Before we start, what is ClinicalTrials.gov?
ClinicalTrials.gov at its core is an updated “registry and results database” regarding clinical studies. People can register to participate in a clinical trial on this website and also review the submitted results after the study ends. The site is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH). Their goal is to “provide patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions.”
Navigating ClinicalTrials.gov
In this article we’ll discuss how to find and view clinical studies and how to learn more about clinical research!
1. Find and view clinical studies
On the home page of ClinicalTrials.gov you will find the below search box. Filling out all the indications will help narrow or widen your search for a clinical trial.
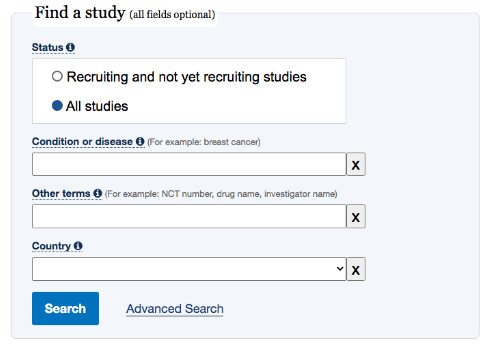
We recommend using the Advanced Search button to narrow your search further. Here you will be able to target your search, specify eligibility criteria, location and more.
Once you have clicked ‘Search’, the site will take you to a page with a list of clinical trials based on your search criterias. This page displays studies status, title, conditions, Interventions and locations. When a study’s status is listed as “Recruiting” this indicates that the trial is actively recruiting participants!
Click on ‘Studies Title’ to discover further information. We recommend reading the description, outcome measures, eligibility criteria and location before moving forward. From here, you should be able to find a link to the study’s website or study representative’s contact information. Reach out and see if you qualify!
Additionally, under the ‘Find Studies’ Tab you can view studies by topic, on a map and even search results for clinical trials.
To learn even more about how to search for studies on ClinicalTrials.gov here.
2. Learn more about clinical research:
Click on ‘About Studies’ to learn more about how clinical studies are conducted and who can participate.

Click on ‘Learn About Studies’ for answers to the below questions:
What Is a Clinical Study?
Who Conducts Clinical Studies?
Where Are Clinical Studies Conducted?
How Long Do Clinical Studies Last?
Reasons for Conducting Clinical Studies
Participating in Clinical Studies
Click on ‘Glossary of Common Site Terms’ to reference words and phrases that are frequently used in clinical studies. . Here are a few terms we find most helpful:
Phase – The stage of a clinical trial studying a drug or biological product, based on definitions developed by the U.S. Food and Drug Administration (FDA). The phase is based on the study’s objective, the number of participants, and other characteristics. There are five phases: Early Phase 1 (formerly listed as Phase 0), Phase 1, Phase 2, Phase 3, and Phase 4. Not Applicable is used to describe trials without FDA-defined phases, including trials of devices or behavioral interventions.
Observational study – A type of clinical study in which participants are identified as belonging to study groups and are assessed for biomedical or health outcomes. Participants may receive diagnostic, therapeutic, or other types of interventions, but the investigator does not assign participants to a specific interventions/treatment. A patient registry is a type of observational study.
Interventional study (clinical trial) – A type of clinical study in which participants are assigned to groups that receive one or more intervention/treatment (or no intervention) so that researchers can evaluate the effects of the interventions on biomedical or health-related outcomes. The assignments are determined by the study’s protocol. Participants may receive diagnostic, therapeutic, or other types of interventions.
Principal investigator (PI) – The person who is responsible for the scientific and technical direction of the entire clinical study.
Protocol – The written description of a clinical study. It includes the study’s objectives, design, and methods. It may also include relevant scientific background and statistical information.
References:
Glossary of Common Site Terms. ClinicalTrials.gov. (n.d.). Accessed October 7, 2021, from https://clinicaltrials.gov/ct2/about-studies/learn#ClinicalTrials.
Learn about clinical studies. ClinicalTrials.gov. (n.d.). Accessed October 7, 2021, from https://clinicaltrials.gov/ct2/about-studies/learn#ClinicalTrials.






